
Med Communications is a dynamic leader with unmatched expertise supporting pharmaceutical and biotech industry partners in medical and scientific communications offering the following: Client-Centric Global Support Our global company with…

When your business spans the globe, one of the challenges of providing medical information support is that your customers’ business hours don’t always align with your own. Med Communications has…

Preparing for a rare disease or orphan drug launch? Let Med Communications customize a launch team for you. Our scientific writers, 90% of whom hold PharmD degrees, are highly educated…
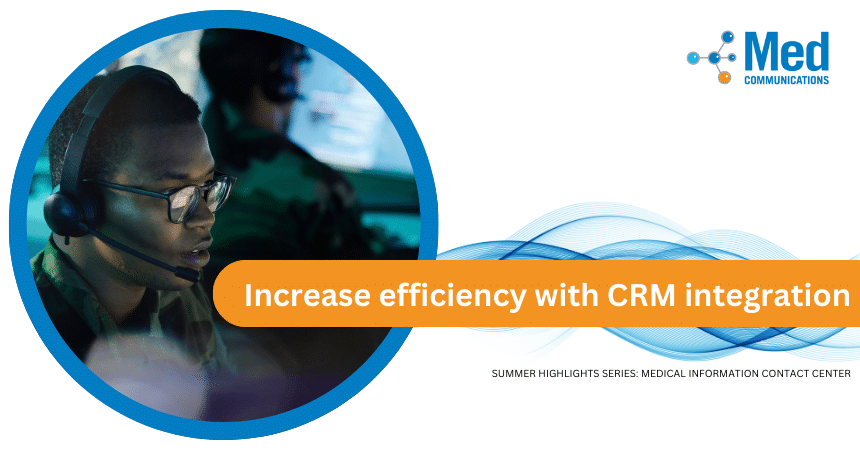
Pharmaceutical and biotechnology companies can receive Medical Information Requests from a variety of sources. Many companies use a Customer Relationship Management (CRM) system such as Veeva to support their field…
On-time vaccination is critical to help control the spread of preventable diseases. Med Communications has more than 10 years of experience covering vaccines, and proudly supports a large percentage of…
Med Communications is a leading expert with a state-of-the-art global medical information contact center. Over 25 Years’ Experience Med Communications has been a trusted partner and resource in providing medical…
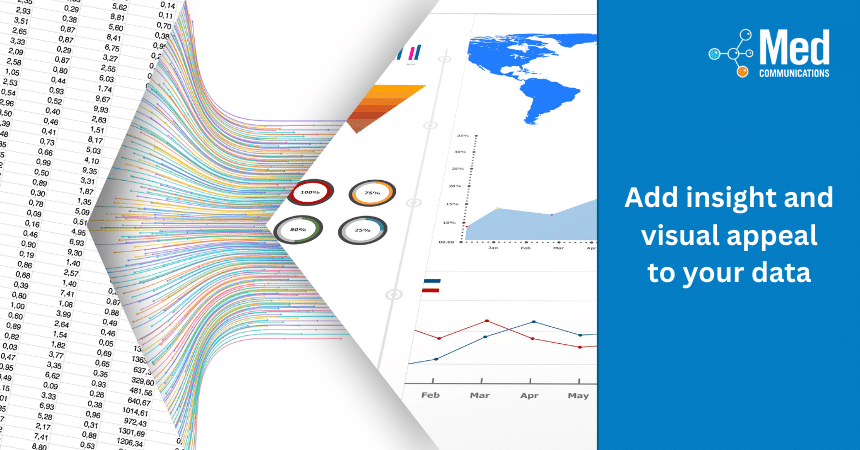
Infographics are a visual representation of data, and an effective tool to present key information to an audience in a clear and concise way. Infographics typically combine different elements such…

Med Communications wishes our American colleagues a happy 4th of July, and hope you have a fun, safe celebration with family and friends. We offer contact center support 24 hours a day, 365 days…

The United States Food and Drug Administration (US FDA) is now accepting investigational new drug application (IND) individual case safety reports (ICSRs) submitted electronically via the Electronic Submission Gateway in ICH…

Communication is key to a successful vendor-client relationship. Expressing your expectations of the vendor from the outset and providing detailed feedback and updates throughout the project can improve workflow and…









