
Customer communication in the pharmaceutical industry looks much different than it did just a decade ago. Digital innovations have brought us tools such as interactive standard responses, which offer an…

Managed care organizations and health care decision makers rely on AMCP formulary dossiers when making formulary and medical policy coverage or reimbursement decisions. These dossiers include the most recent, relevant,…

In today’s evidence-rich but fast-paced research environment, the challenge is to ensure your publications communicate value with clarity, accuracy, and ethical rigor. Too many strong datasets still underperform because the…

Med Communications joins in the recognition of women in the medical, pharmaceutical, and other scientific fields in honor of the International Day of Women and Girls in Science tomorrow, February…

Check out our latest video, in which our president and CEO Allen Scoggin provides an overview of Med Communications and our growth over the past quarter century into a global…

Is your Medical Information Contact Center vendor too big to serve your company well? Here are some telltale signs. Are you experiencing any of these challenges in your medical information…

Med Communications’ Global Head of Pharmacovigilance Maria Anitan, Business Development Lead Stacy Witham, and Business Development Representative Natalie McClendon will be attending the DIA Global Pharmacovigilance and Risk Management Strategies…

Literature searches offer pharmaceutical and biotech companies support for scientific, medical, and regulatory content creation. These searches serve as a strategic foundation for shaping scientific communication and guiding medical affairs…
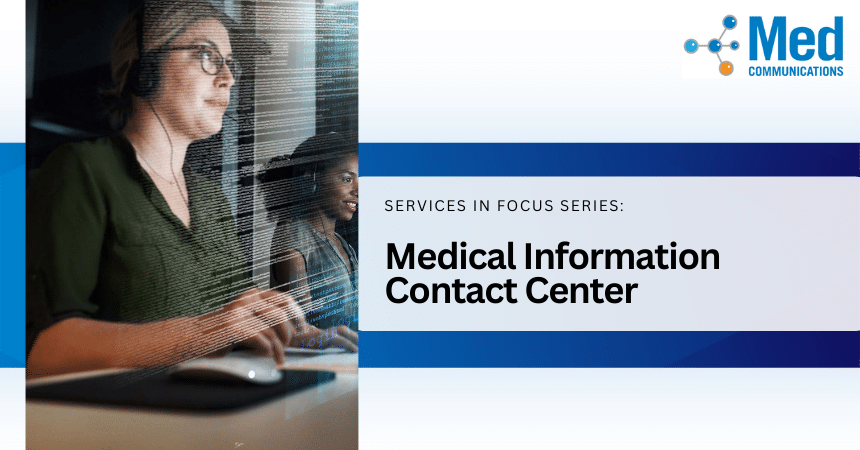
The contact center is the epicenter for customer service for many companies in the life sciences industry, serving as the hub for inquiries, concerns, and feedback from patients, caregivers, and…

On National Pharmacist Day, observed annually on January 12th, we honor the nearly 350,000 pharmacists in the United States who promote patient health by dispensing medications, advising patients, and providing…









