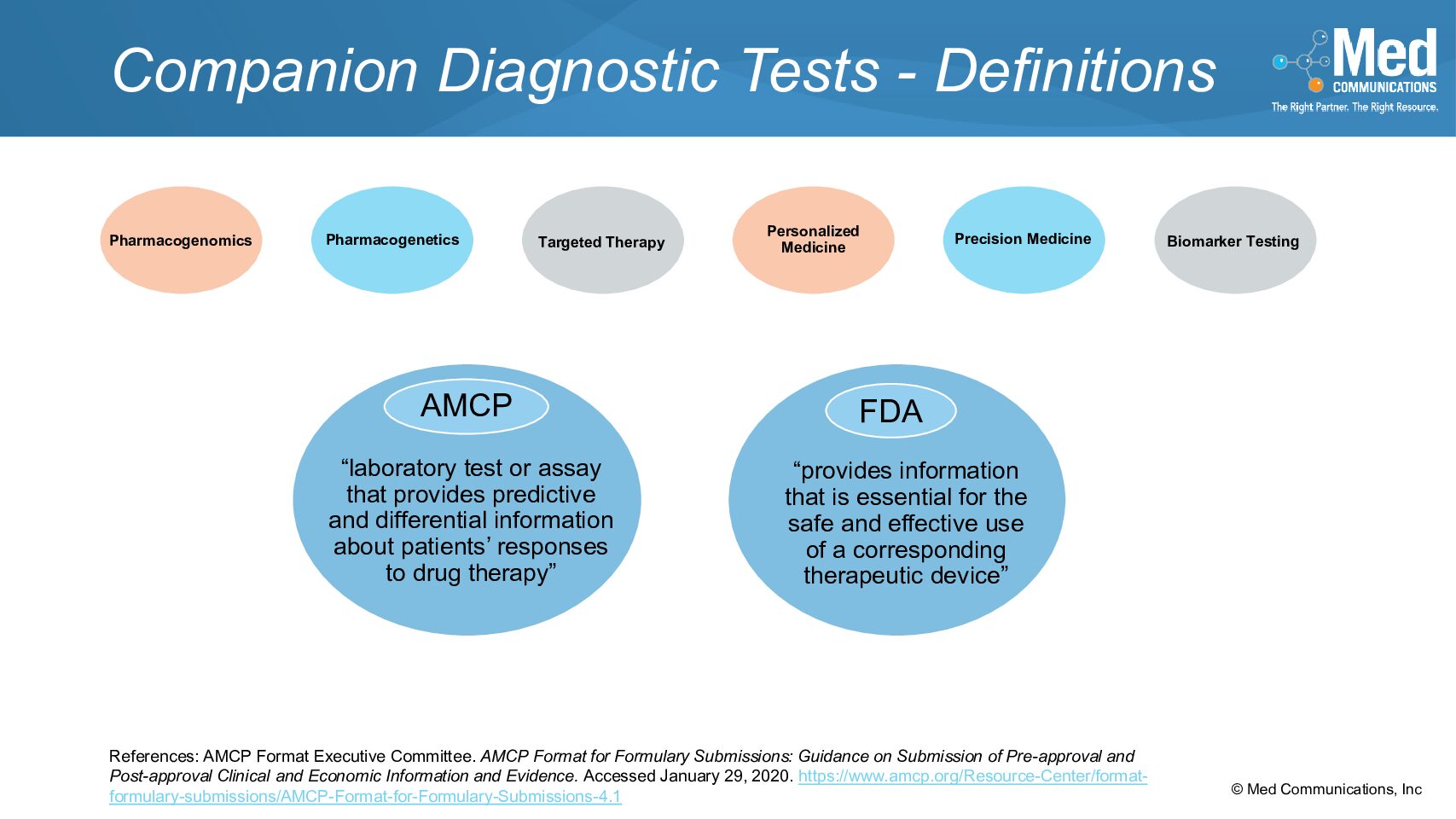
In the Special Content Considerations section of the AMCP Format for Formulary Dossiers, the third topic is Companion Diagnostic Tests. There are many ways to define companion diagnostic tests (CDTs),…

In the Special Content Considerations section of the AMCP Format for Formulary Dossiers, the second topic is Dossier for Clinical Laboratory Tests and Medical Devices. While the AMCP format can…
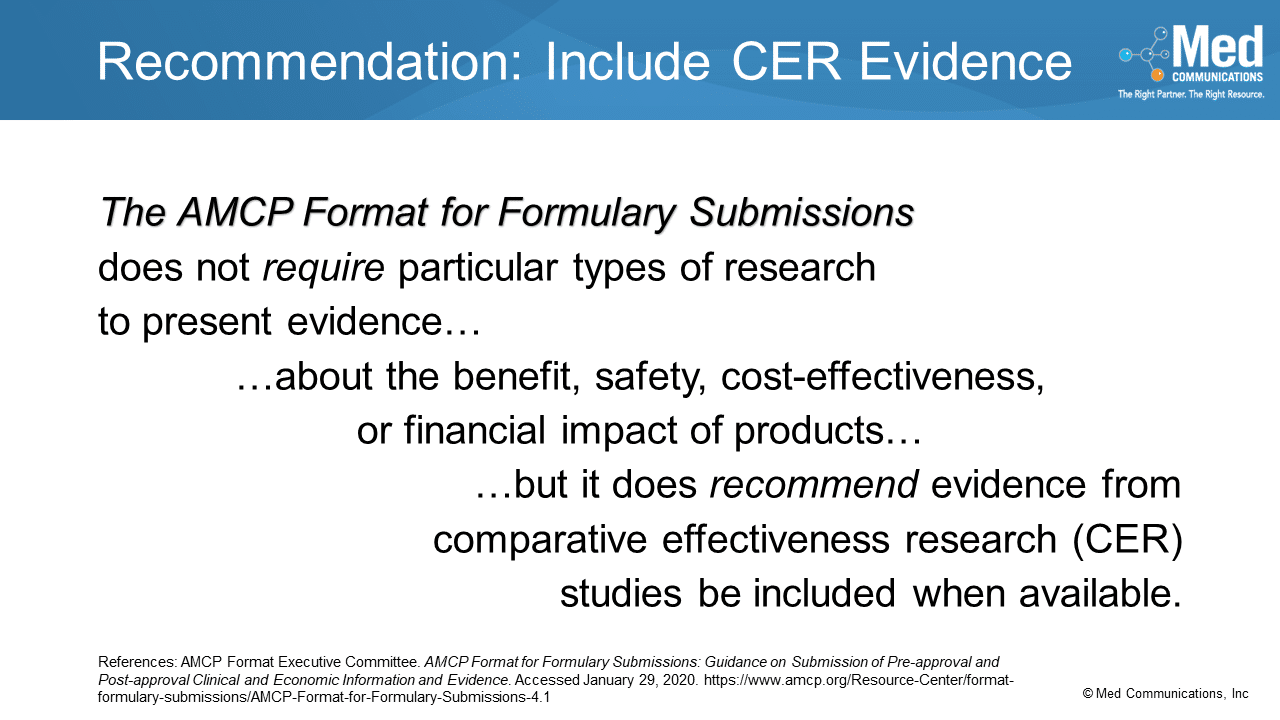
In the Special Content Considerations section of the AMCP Format for Formulary Dossiers, the first topic is Comparative Effectiveness Research. While most manufacturers provide evidence from randomized, controlled trials as…

Med Communications has a high percentage of workers who normally work from their own home offices. We believe this allows us to get the best people for the job and…

During these complex and trying times, we are here for you, the client, the patient, and each other. If your company is in need of additional support in medical call…

At Med Communications, we specialize in presenting high-quality, timely, and accurate scientific information, so we appreciate getting facts from scientific experts. If you’re looking for high-quality and accurate scientific information…

The DIA Medical Affairs and Scientific Communications Forum has been moved to May 6-7, 2020 and will be a virtual event, with both speakers and attendees participating remotely via a…

Are you attending the DIA Medical Affairs and Scientific Communications Forum next week? You’ve probably heard it’s gone virtual, but for our Director of Medical Communications, Ellen Whipple, the show…
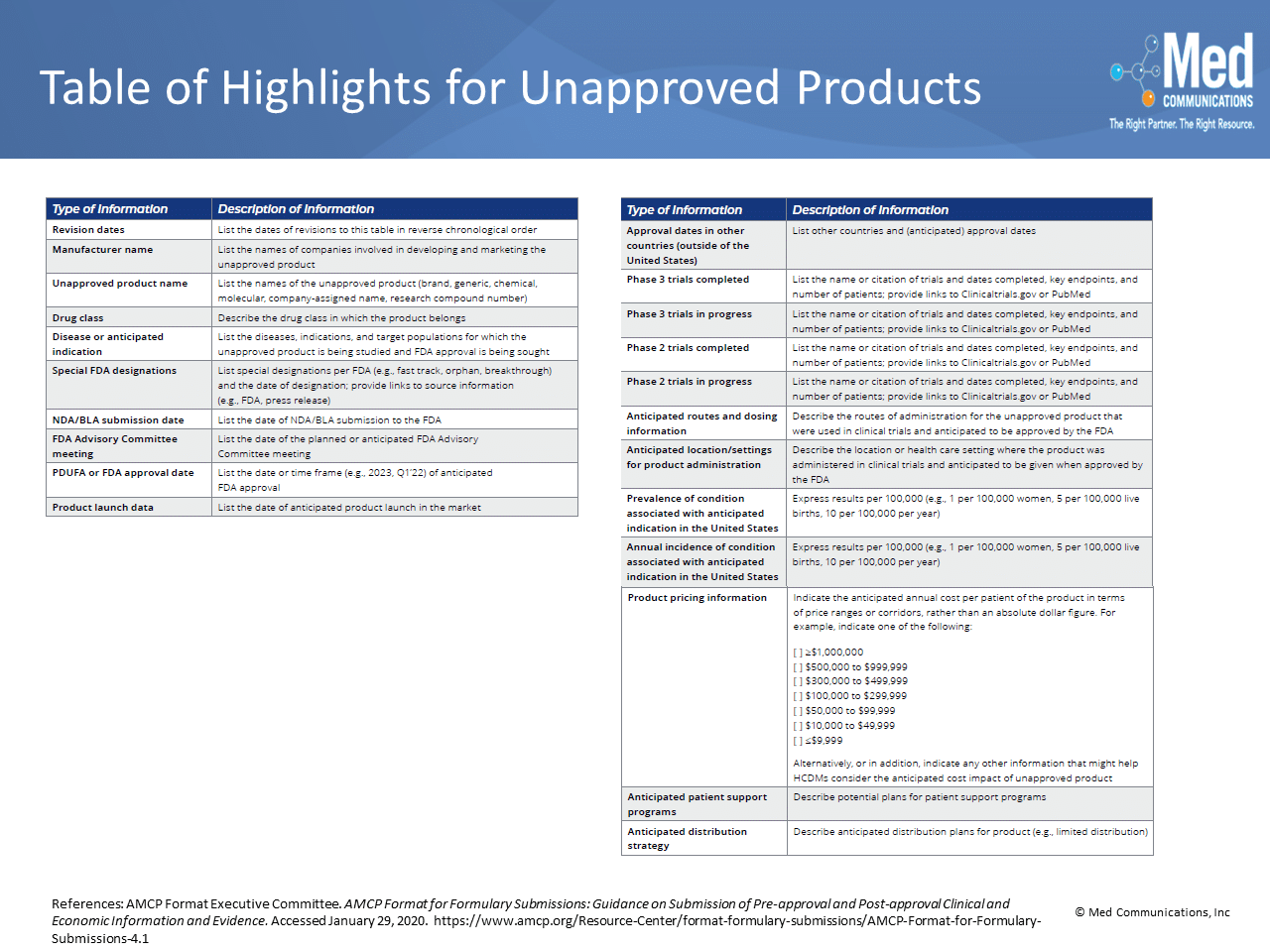
The formats for Unapproved Product and Unapproved Use Dossiers are new with the latest AMCP Format for Formulary Dossiers. Because manufacturers cannot make claims about an unapproved product or use,…
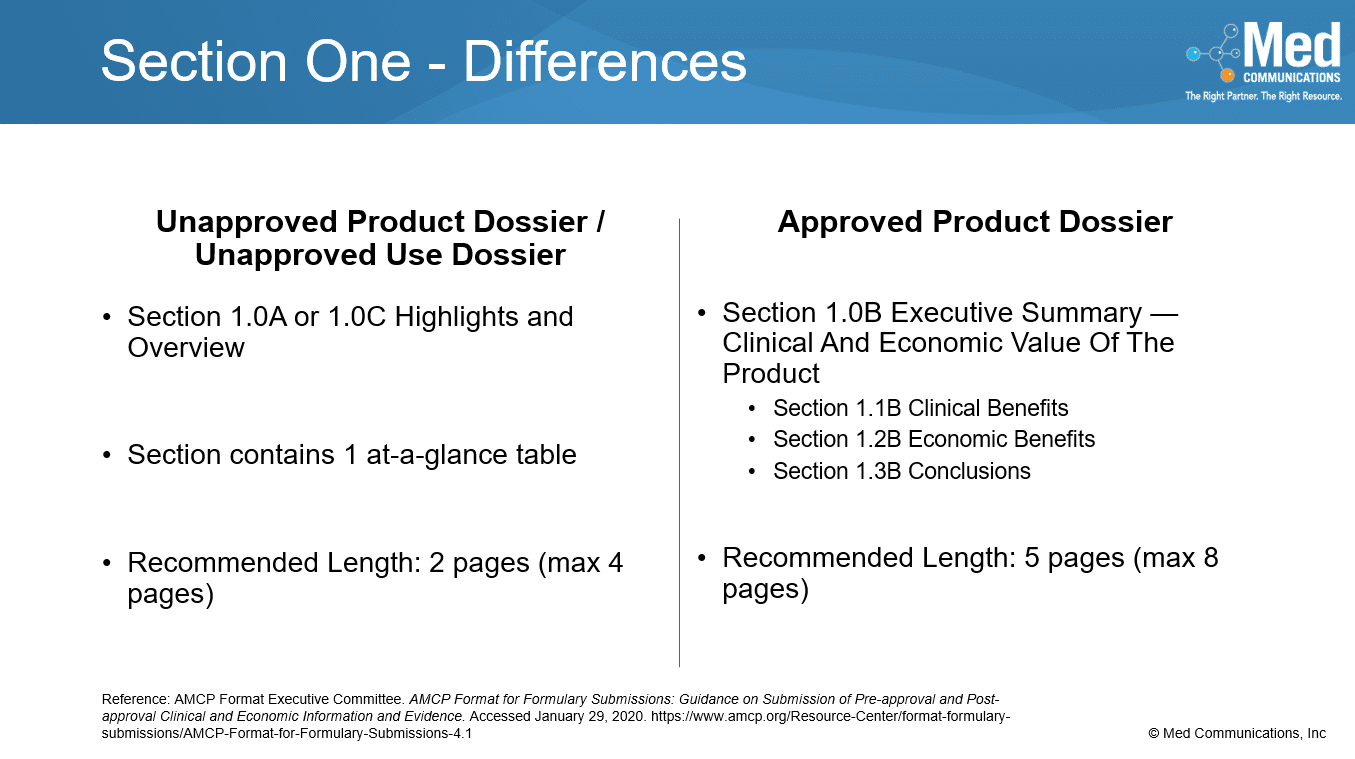
While there are some similarities between the sections in the Approved Product Dossiers and the Unapproved Product and Unapproved Use Dossiers, there are many differences. Section 1 provides a different…








