
A pharmacovigilance (PV) system covering all PV activities is required of all marketing authorization holders. This system must include the activities of collection and processing of safety information, reporting to…

Med Communications has benefited from the extensive skills, knowledge, and insights of Business Development Lead Jill Kembel, PharmD, for nearly a decade. Jill has more than 20 years’ experience in…

Every clinical trial requires safety monitoring and management, beginning early in the product development life cycle. The data obtained during safety monitoring help with product safety profile classification and also…

As our American colleagues and clients prepare to celebrate Thanksgiving this Thursday, we wanted to give thanks to each of you. We appreciate your trust and your support — we…

Promotional review is traditionally accomplished by groups of professionals who meet a few times per week to review assets. It generally takes several weeks to approve an asset, and sometimes…

AMCP formulary dossiers offer a comprehensive view of the US clinical, safety, and economic evidence for new and existing medical drugs, tests, and devices, helping health care decision makers make…

Pharmaceutical companies operate in a highly regulated environment, especially when it comes to global pharmacovigilance (PV) systems. Pharmacovigilance professionals play a crucial role in protecting patient safety while navigating complex…

Pharmaceutical and biotech companies often use a rigorous process to select a contact center vendor. Quality, service, experience, speed of implementation, resilience, and cost are some of the many factors…
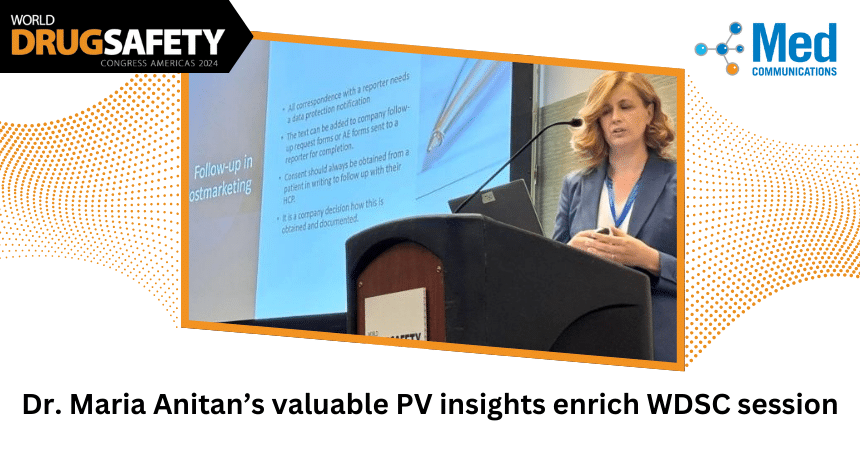
Important pharmacovigilance topics were discussed during the World Drug Safety Congress Americas in Boston last week, including valuable insights on data privacy shared by Med Communications’ Maria Anitan, MD. Some…

In today’s rapidly evolving market, adjusting to product support needs while meeting budgetary requirements are among the top global medical affairs concerns. Efficient use of time and resources allows a…









