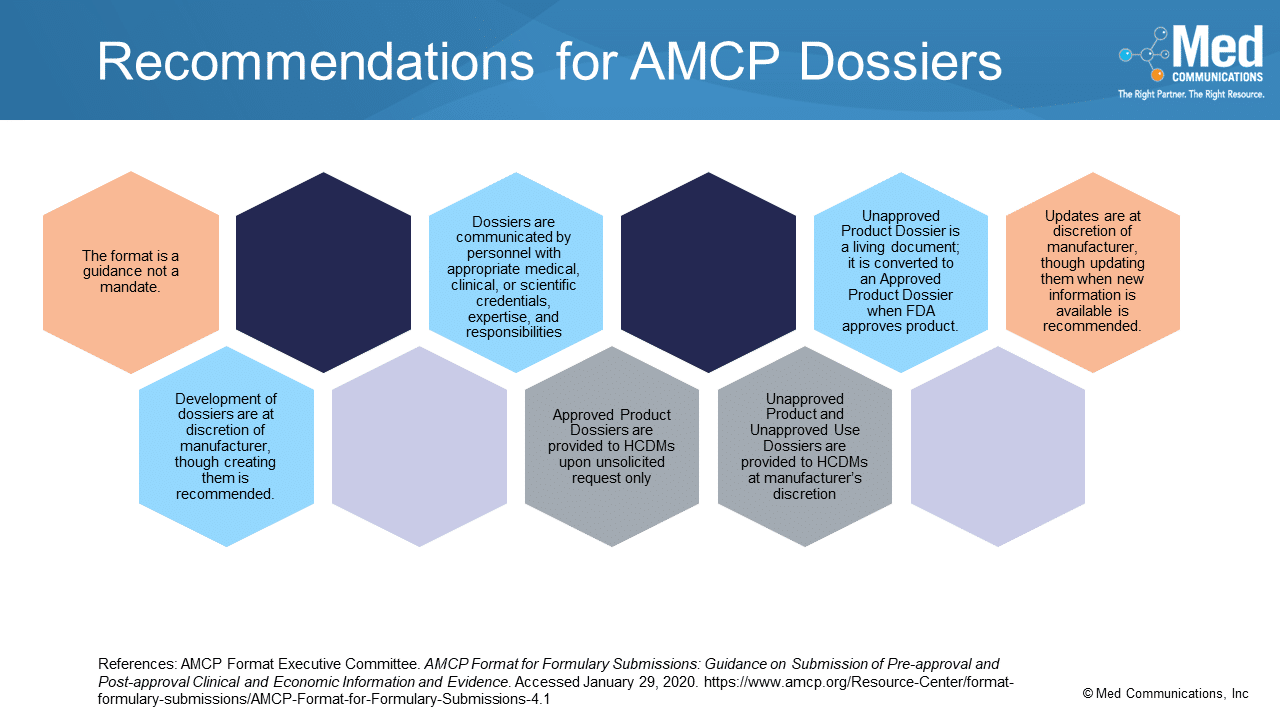
While many organizations follow the AMCP Format for Formulary Submissions, it is only a guidance, not a mandate, for how manufacturers and health care decision-makers can act in the process…

The two biggest differences between versions 4.0 and 4.1 of the dossier formats are the addition of the new types of dossiers (discussed in previous posts), and the importance placed…
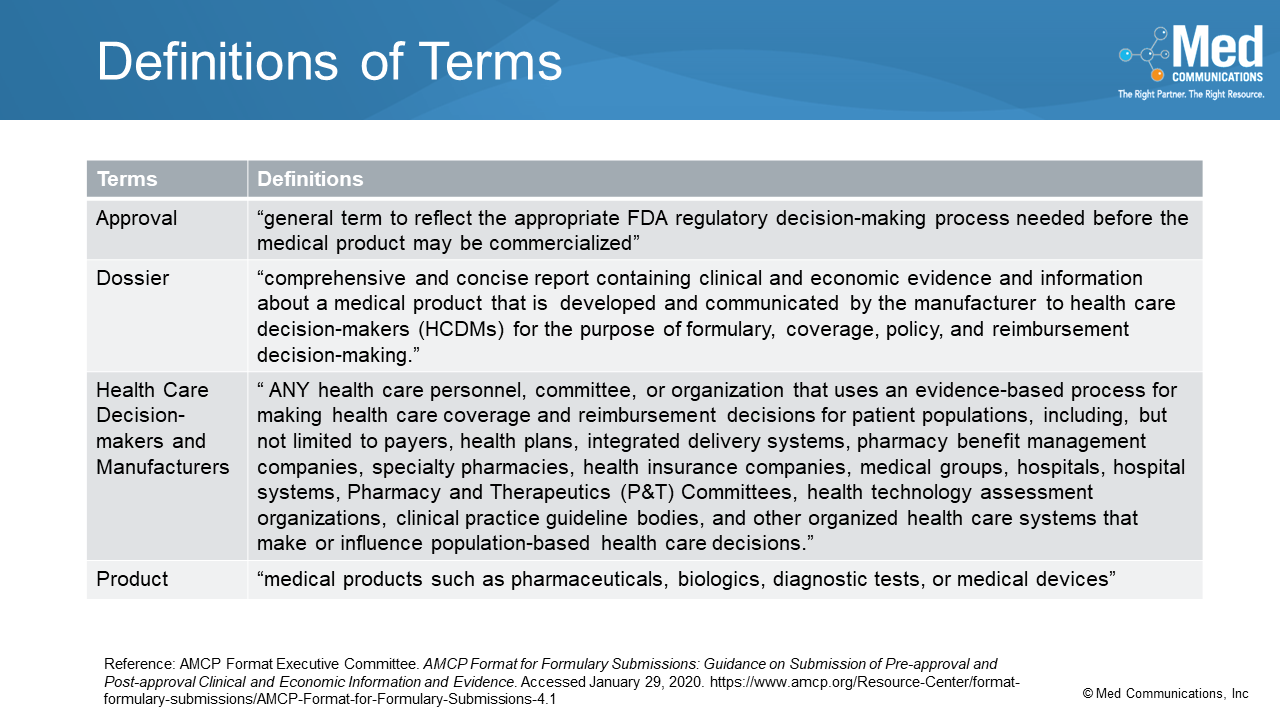
Some of the terms used in the AMCP format have been broadened with the new version. For example, “product” in past versions primarily meant pharmaceutical products, but that term has…
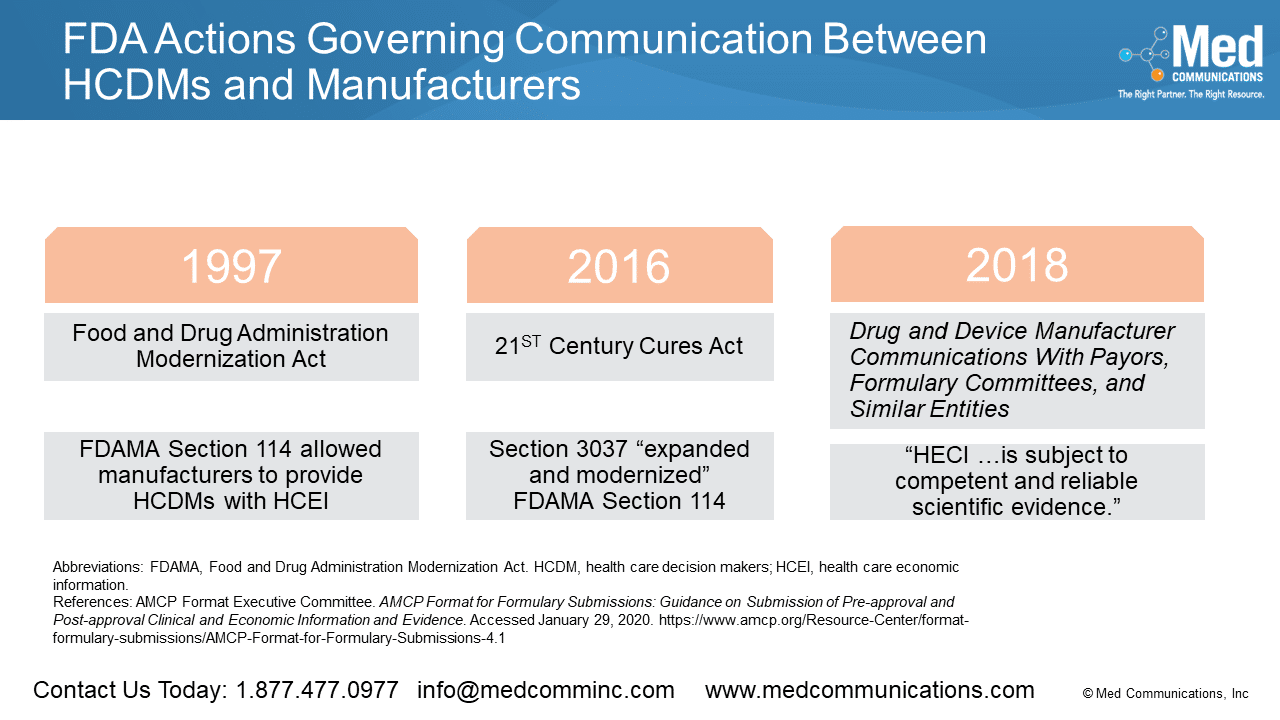
One driver for the new AMCP format was the FDA Final Guidance, Drug and Device Manufacturer Communications With Payors, Formulary Committees, and Similar Entities, which allowed for communication of information…
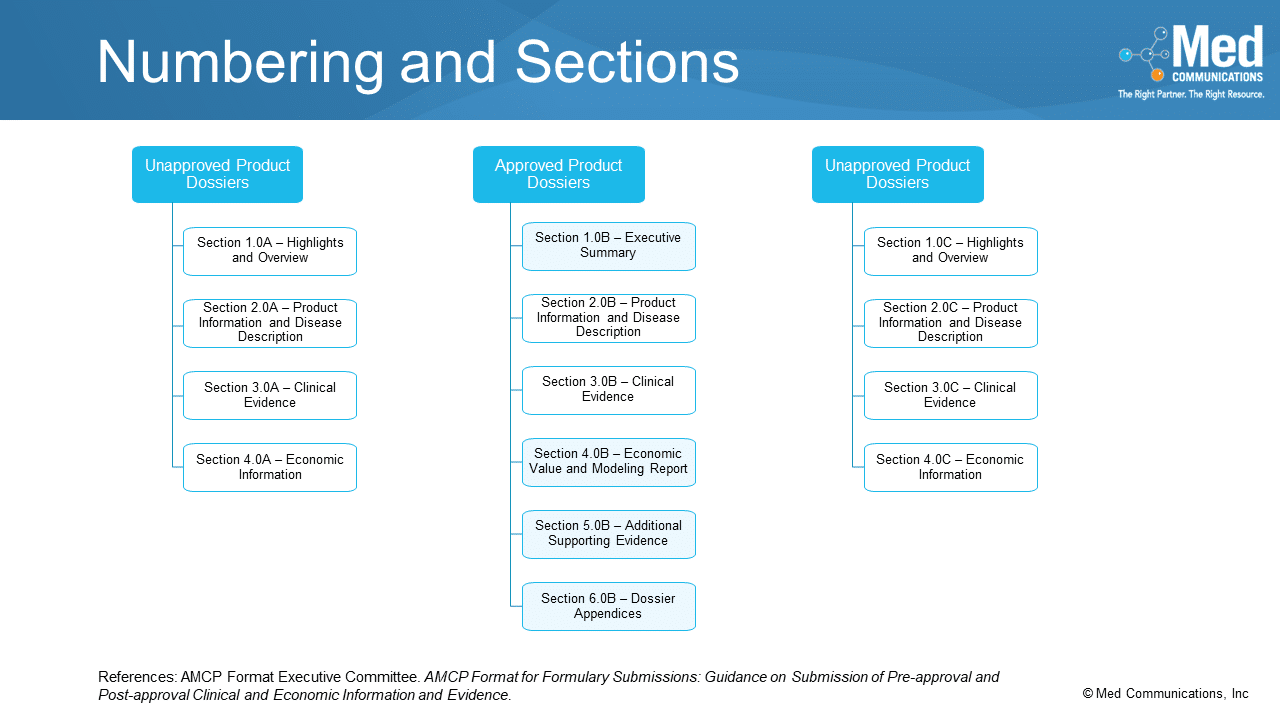
With the change to having three types of formulary dossiers in the new AMCP Format for Formulary Submissions, the numbering of the sections within each dossier changes to include an…
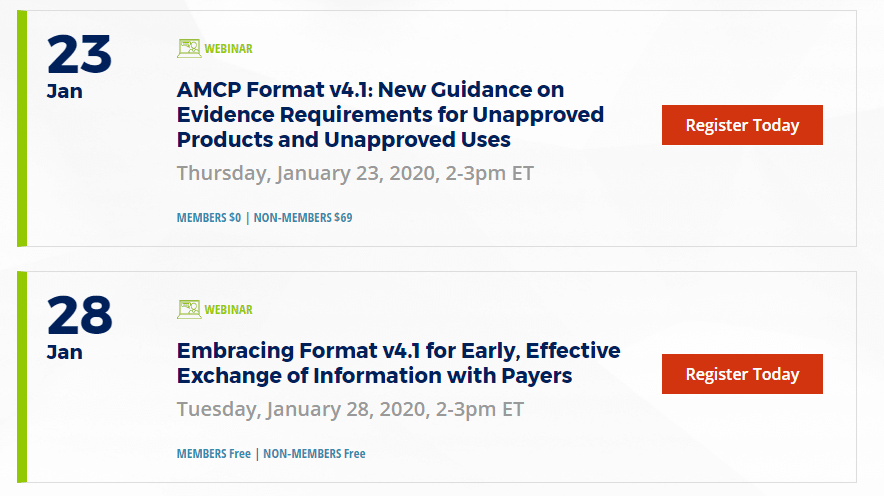
If you’re wondering how the new dossier format affects your company’s or your client’s formulary dossiers, you have a few options to get up to speed: ✓ Follow our page…

One big change in the new AMCP Format for Formulary Submissions is an addition of two different types of dossiers and new terminology to describe them. The “traditional” dossier that…

A week ago, our Director of Medical Communications, Ellen Whipple, BPharm and PharmD, was on a panel on about the new AMCP Format Version 4.1, which had just been released…
AMCP Nexus is dedicated to bringing the many facets of managed care pharmacy and health care together to plan for the coming wave of changes in our work and our…
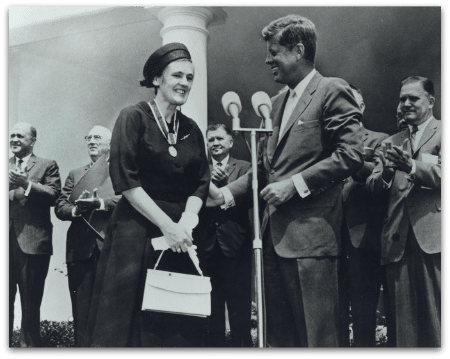
In August, Dr. Frances Oldham Kelsey passed away, and she is important to history because she is regarded as the heroine that saved the US from the Thalidomide tragedy that…








