
In an Unapproved Product Dossier, the information in some of the sections is different than what’s included in those sections in an Approved Product Dossier. Because the product is not…

The new European Union (EU) Medical Devices Regulation (MDR), which was supposed to become fully active on May 26th, 2020, has been postponed for 1 year, in light of the…
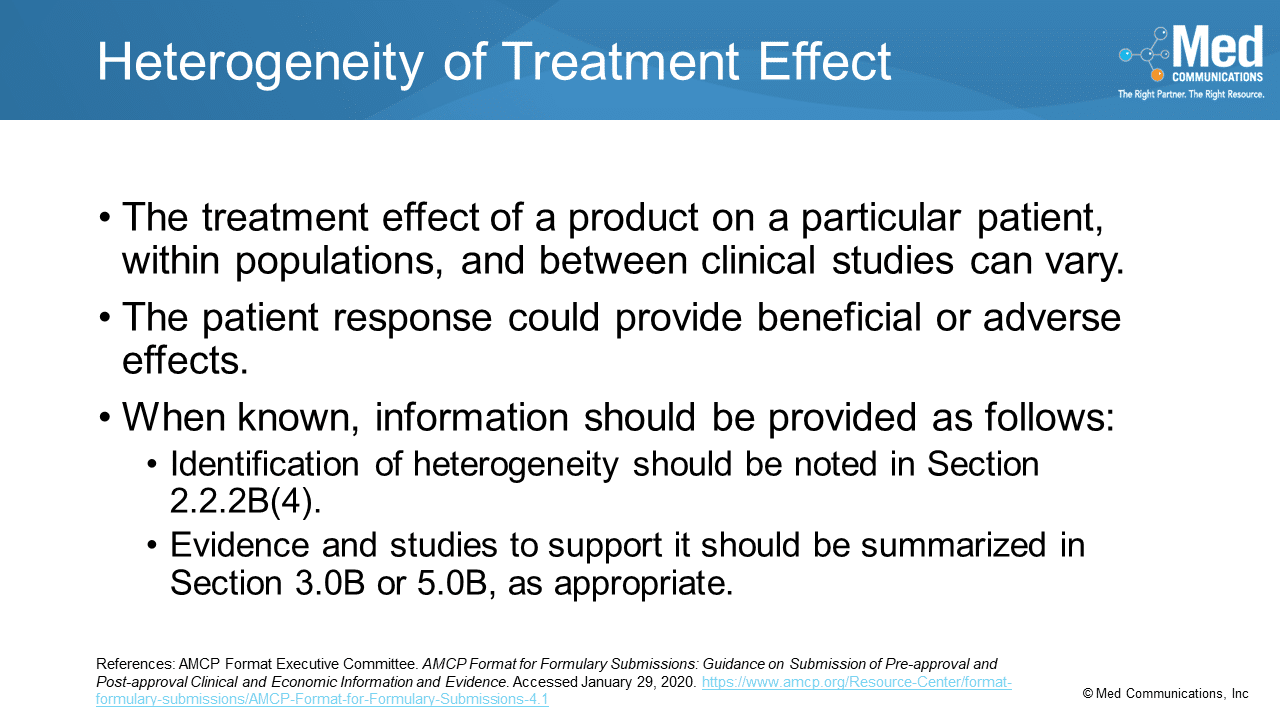
In the Special Content Considerations section of the AMCP Format for Formulary Dossiers, the last topic is Heterogeneity of Treatment Effect. Health care decision makers need to know how certain…
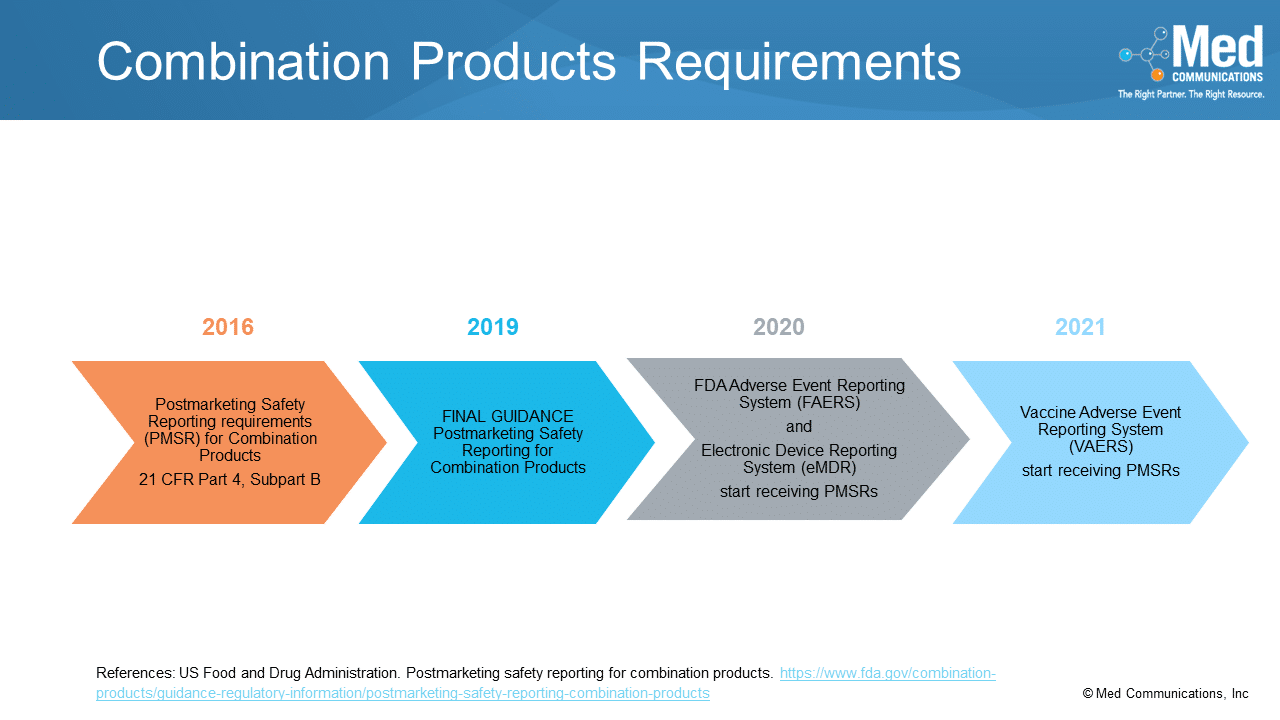
In recent years, we have witnessed an increase in patients’ expectations for more innovative approaches from pharmaceutical companies in developing medical products that are easy to use, deliver the correct…
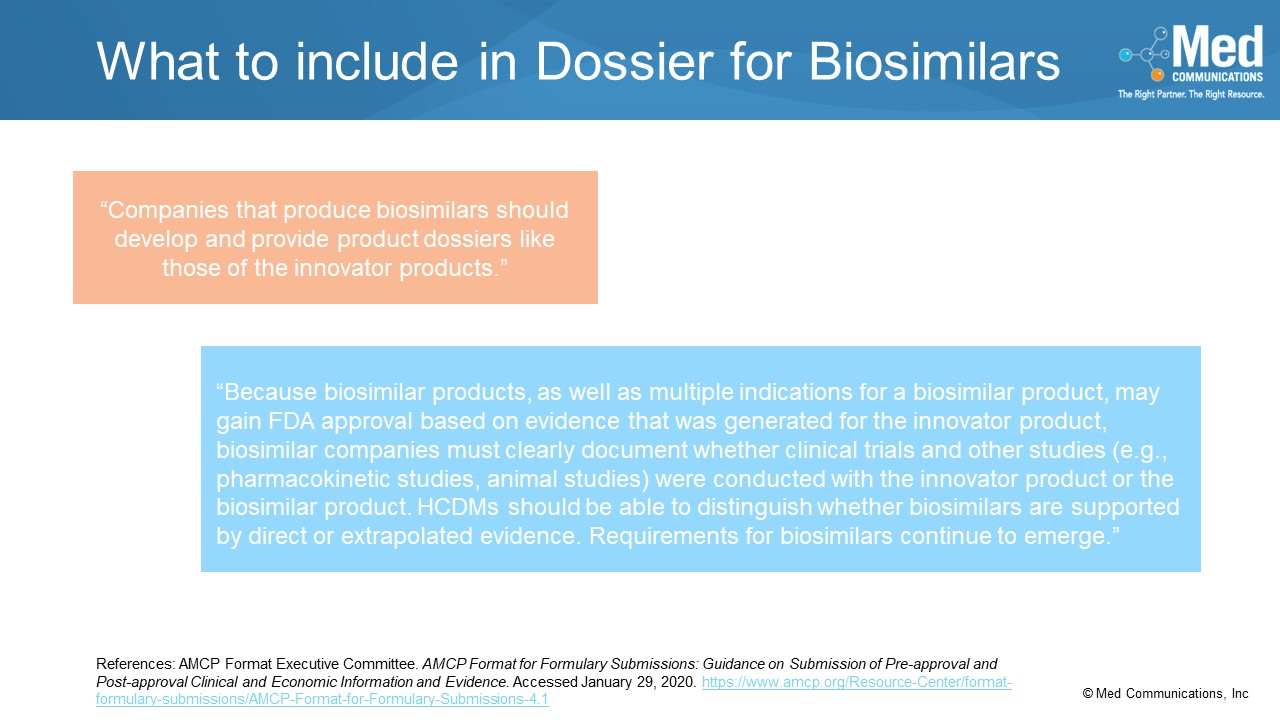
In the Special Content Considerations section of the AMCP Format for Formulary Dossiers, the fourth topic is Biosimilars. With the interest in biosimilars, health care decision makers need to know…
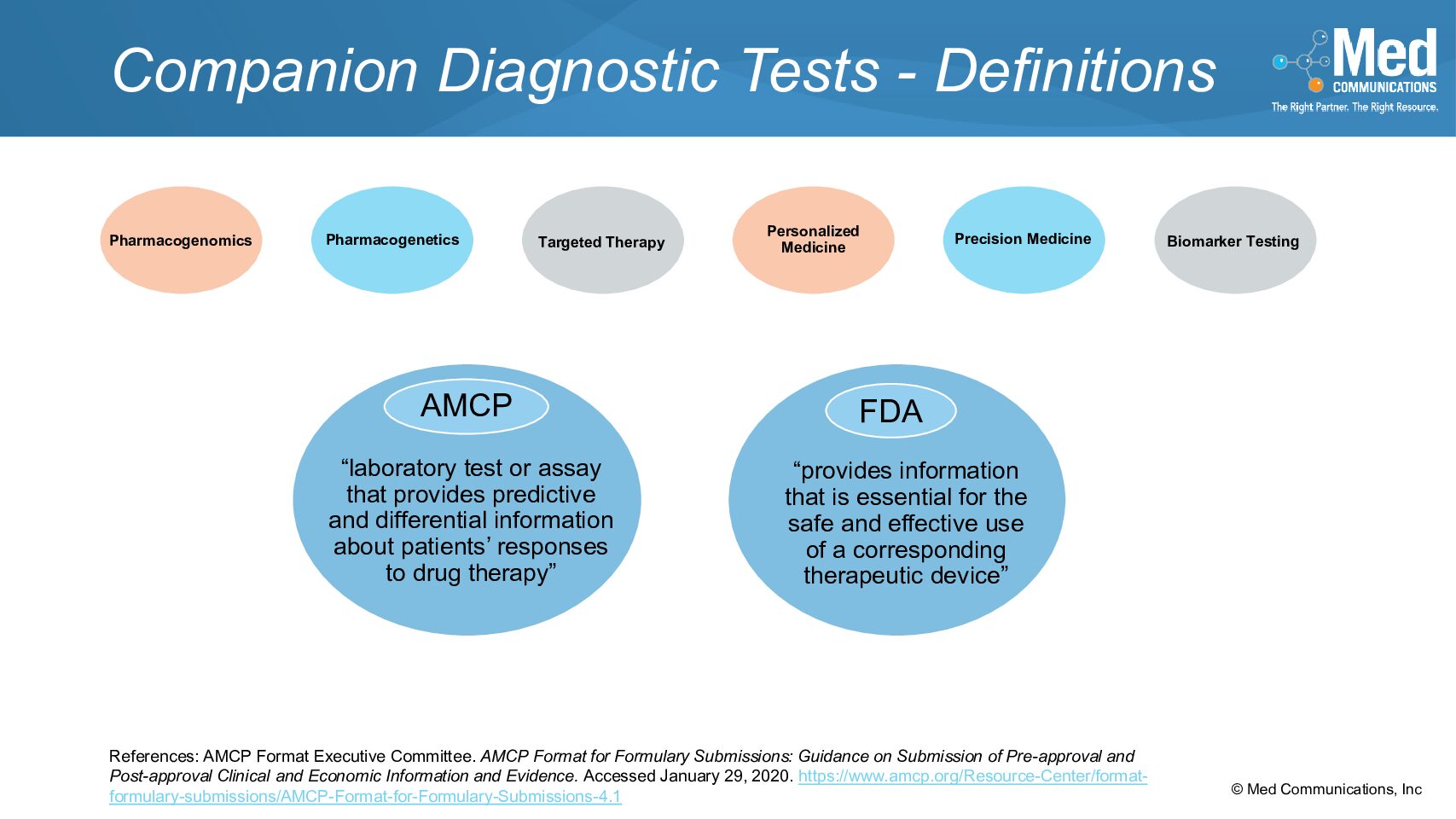
In the Special Content Considerations section of the AMCP Format for Formulary Dossiers, the third topic is Companion Diagnostic Tests. There are many ways to define companion diagnostic tests (CDTs),…

In the Special Content Considerations section of the AMCP Format for Formulary Dossiers, the second topic is Dossier for Clinical Laboratory Tests and Medical Devices. While the AMCP format can…
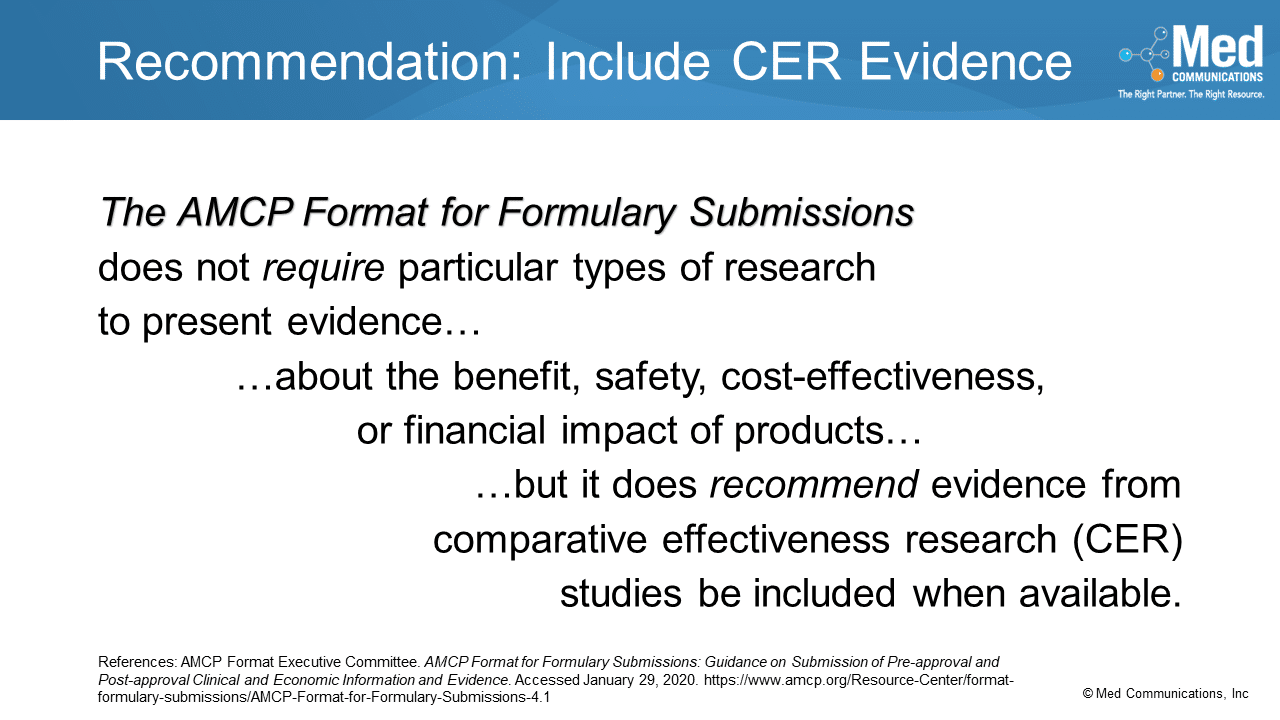
In the Special Content Considerations section of the AMCP Format for Formulary Dossiers, the first topic is Comparative Effectiveness Research. While most manufacturers provide evidence from randomized, controlled trials as…
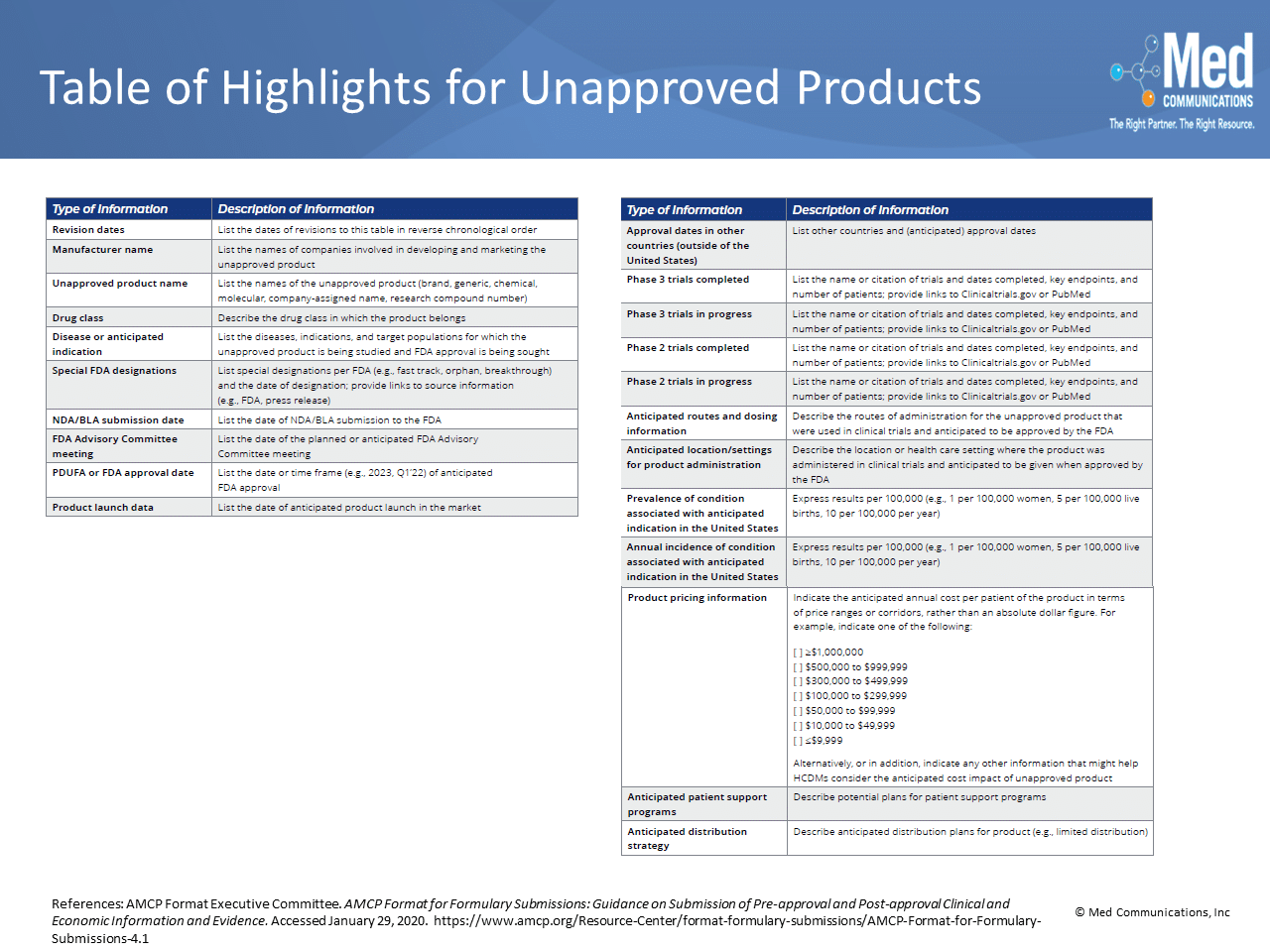
The formats for Unapproved Product and Unapproved Use Dossiers are new with the latest AMCP Format for Formulary Dossiers. Because manufacturers cannot make claims about an unapproved product or use,…
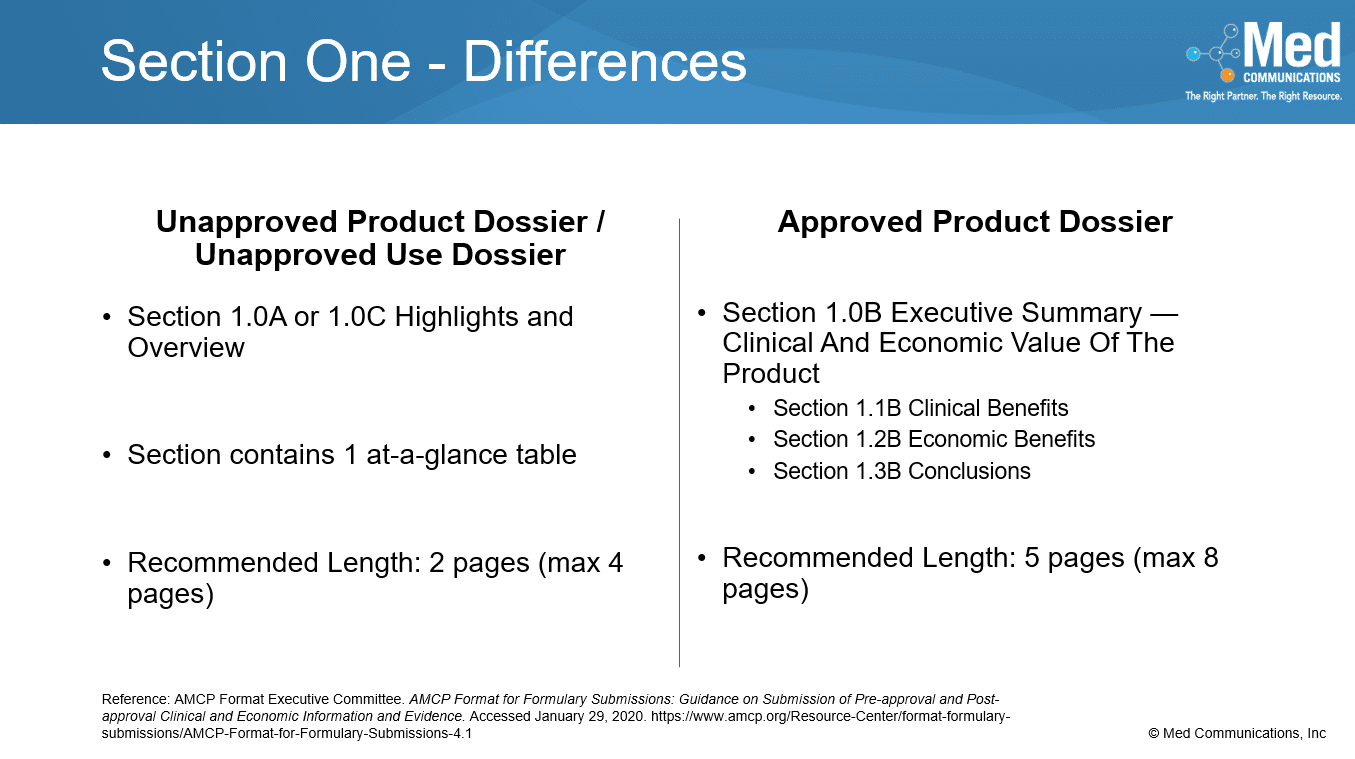
While there are some similarities between the sections in the Approved Product Dossiers and the Unapproved Product and Unapproved Use Dossiers, there are many differences. Section 1 provides a different…









