
The United States Food and Drug Administration (US FDA) is now accepting investigational new drug application (IND) individual case safety reports (ICSRs) submitted electronically via the Electronic Submission Gateway in ICH…

Dealing with safety data overload is nothing new for biotech companies; it has been an ongoing discussion in the industry for many years now. Companies are inundated with post-approval safety…
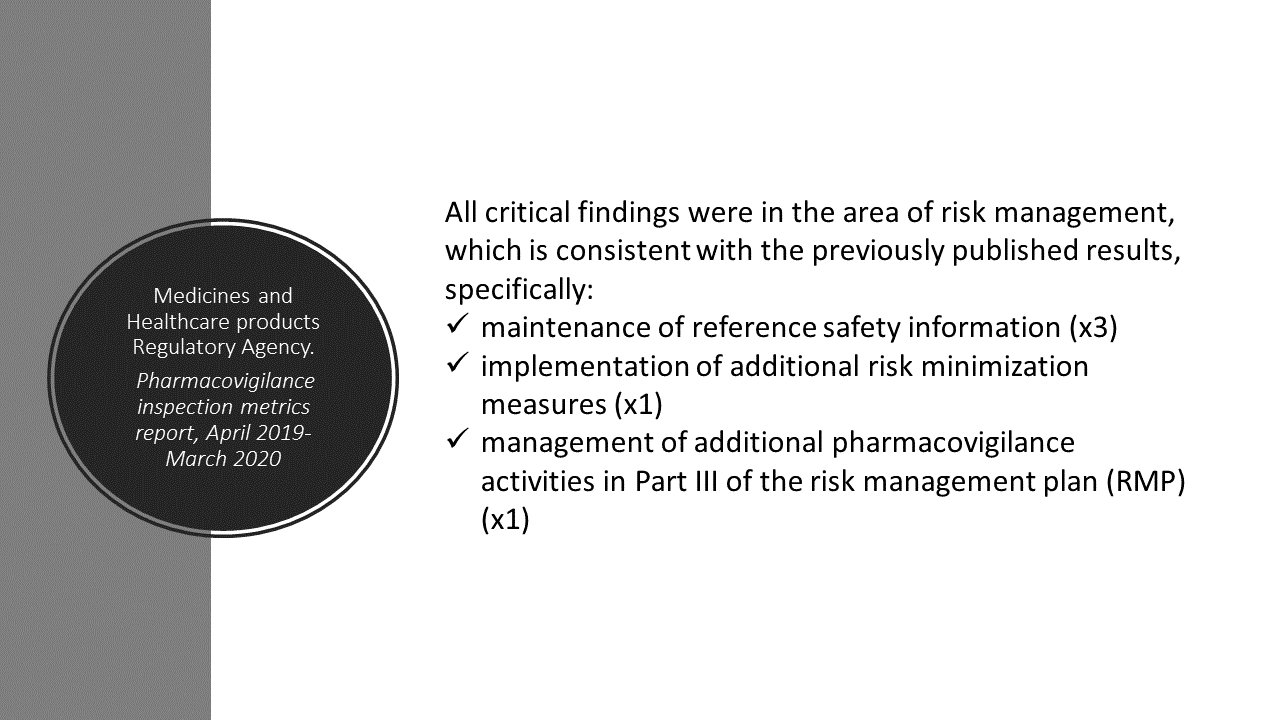
In the latest MHRA GPvP Inspection Metrics Report, risk management comes up as a critical finding. For more information, see post.

In an Unapproved Use Dossier, the information is similar to those sections in an Unapproved Product Dossier. There is some information included about the Approved Product, but this dossier should…

The contents of the Approved Product Dossier do not change in the new AMCP Format for Formulary Dossiers, but there is so much to talk about with this type of…

The contents of the Approved Product Dossier do not change in the new AMCP Format for Formulary Dossiers, but there is so much to talk about with this type of…

The contents of the Approved Product Dossier do not change in the new AMCP Format for Formulary Dossiers, but there is so much to talk about with this type of…

The contents of the Approved Product Dossier do not change in the new AMCP Format for Formulary Dossiers, but there is so much to talk about with this type of…

The contents of the Approved Product Dossier do not change in the new AMCP Format for Formulary Dossiers, but there is so much to talk about with this type of…

The contents of the Approved Product Dossier do not change in the new AMCP Format for Formulary Dossiers; it’s more about the fact that it has a new name to…









