
The contents of the Approved Product Dossier do not change in the new AMCP Format for Formulary Dossiers, but there is so much to talk about with this type of…

The contents of the Approved Product Dossier do not change in the new AMCP Format for Formulary Dossiers, but there is so much to talk about with this type of…

The contents of the Approved Product Dossier do not change in the new AMCP Format for Formulary Dossiers; it’s more about the fact that it has a new name to…

In an Unapproved Product Dossier, the information in some of the sections is different than what’s included in those sections in an Approved Product Dossier. Because the product is not…

The new European Union (EU) Medical Devices Regulation (MDR), which was supposed to become fully active on May 26th, 2020, has been postponed for 1 year, in light of the…
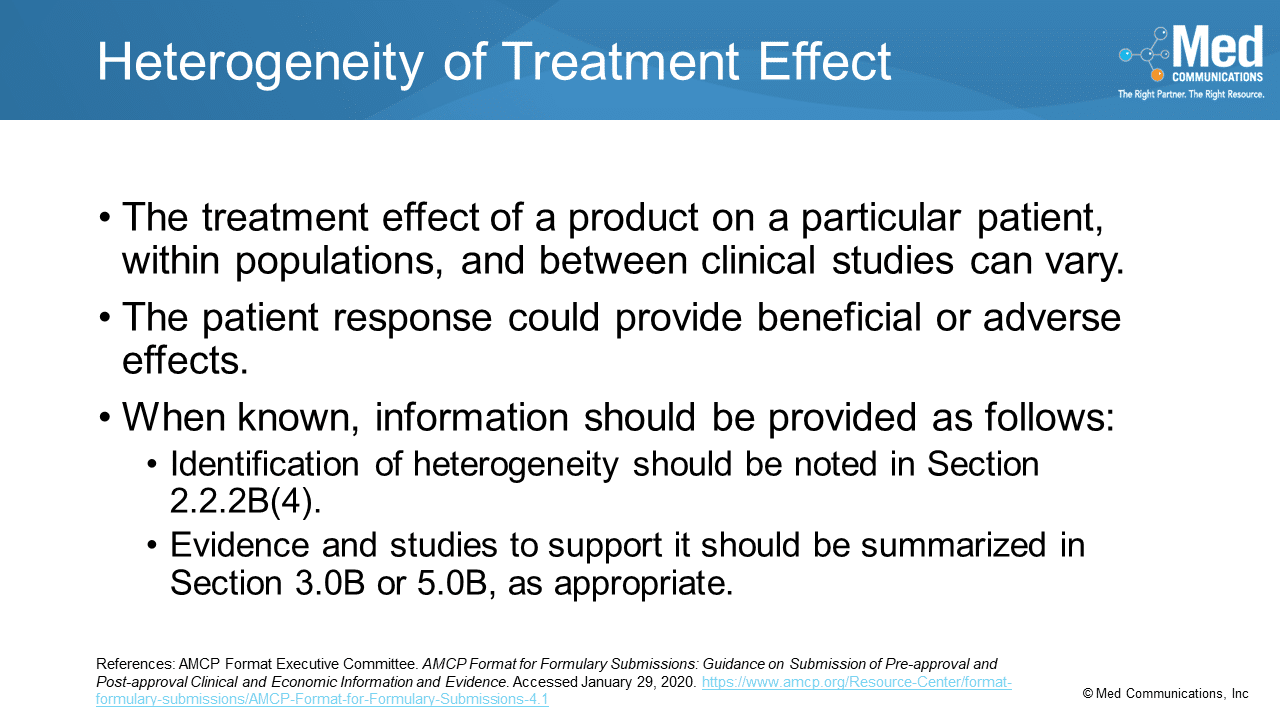
In the Special Content Considerations section of the AMCP Format for Formulary Dossiers, the last topic is Heterogeneity of Treatment Effect. Health care decision makers need to know how certain…
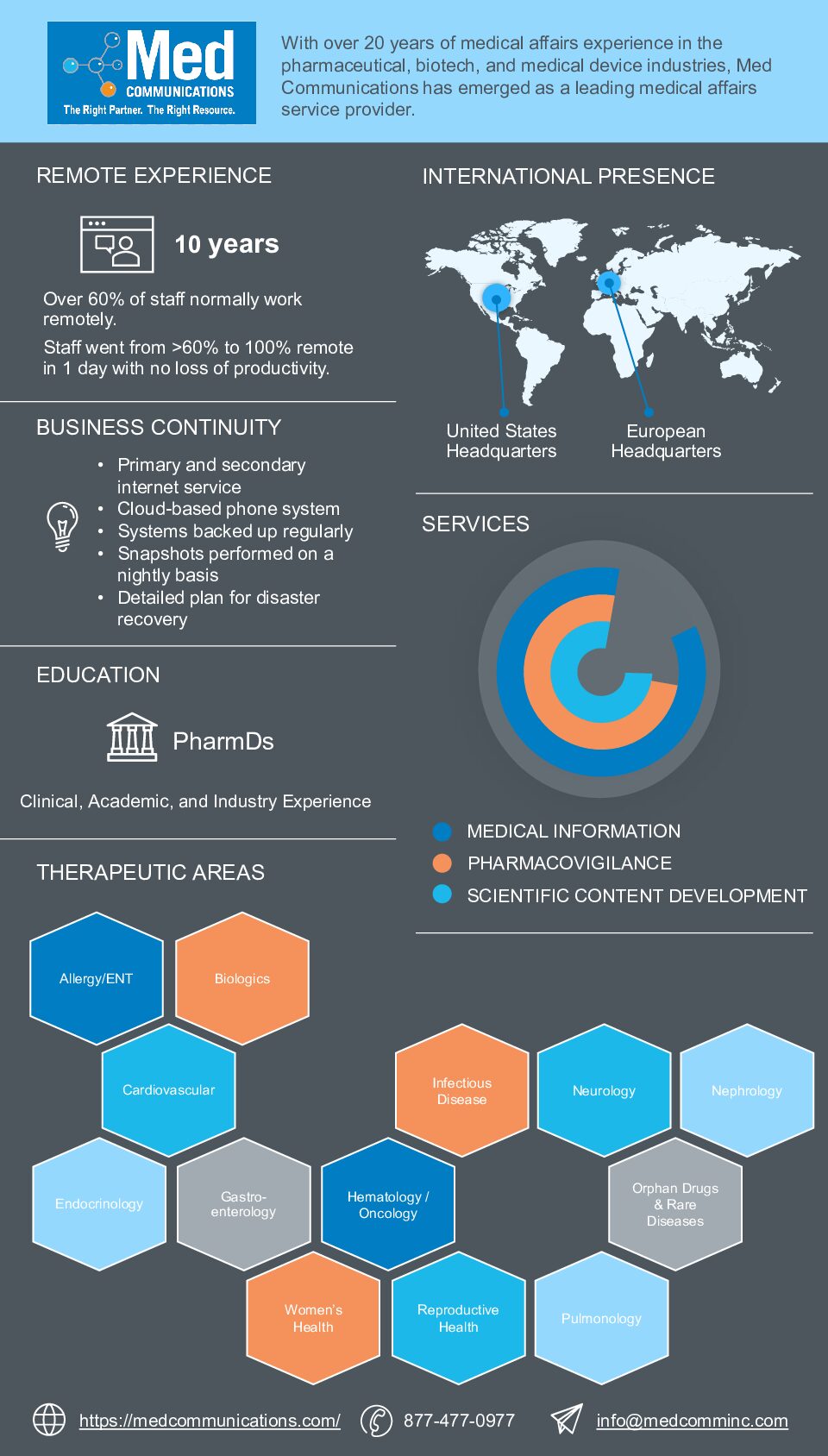
It’s not easy to have a fully remote company, as many companies are realizing. You have to have good systems, great managers, and excellent employees. Med Communications has had a…

Like many in-person conferences this spring, the DIA MASC conference was moved to a virtual format, and it will now take place May 5-7. While the Medical Communications Primer―which included…
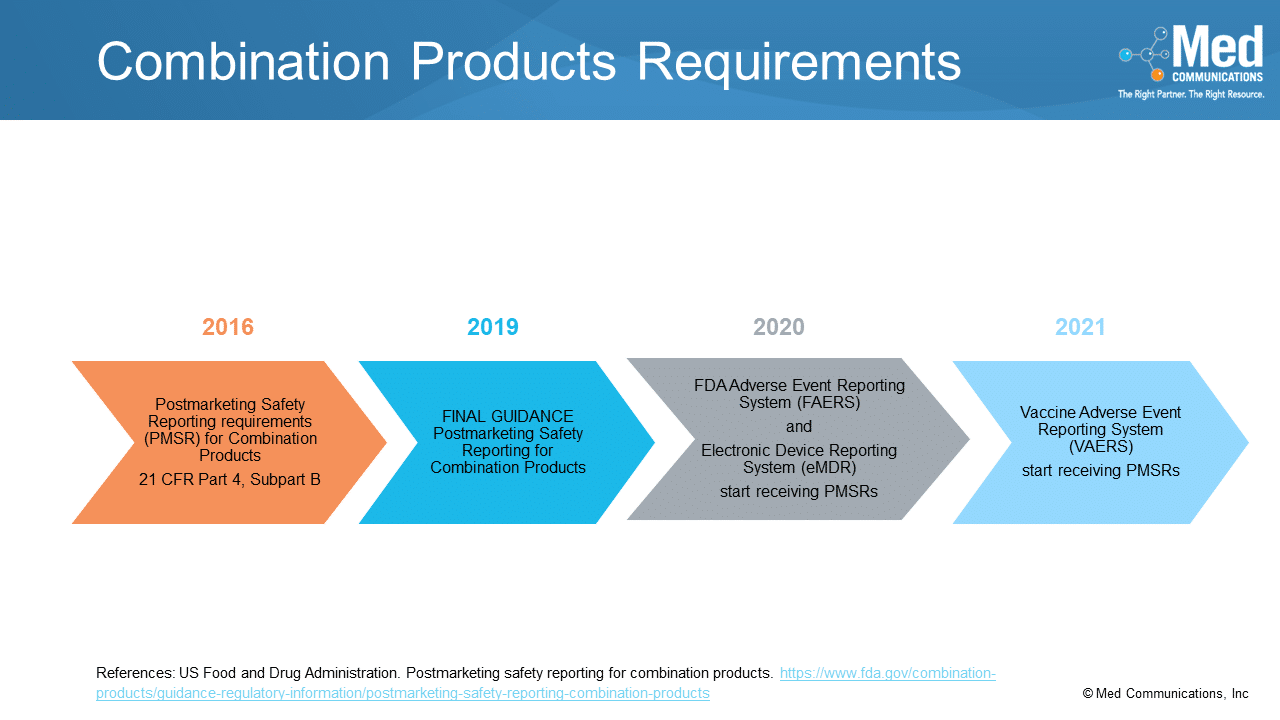
In recent years, we have witnessed an increase in patients’ expectations for more innovative approaches from pharmaceutical companies in developing medical products that are easy to use, deliver the correct…
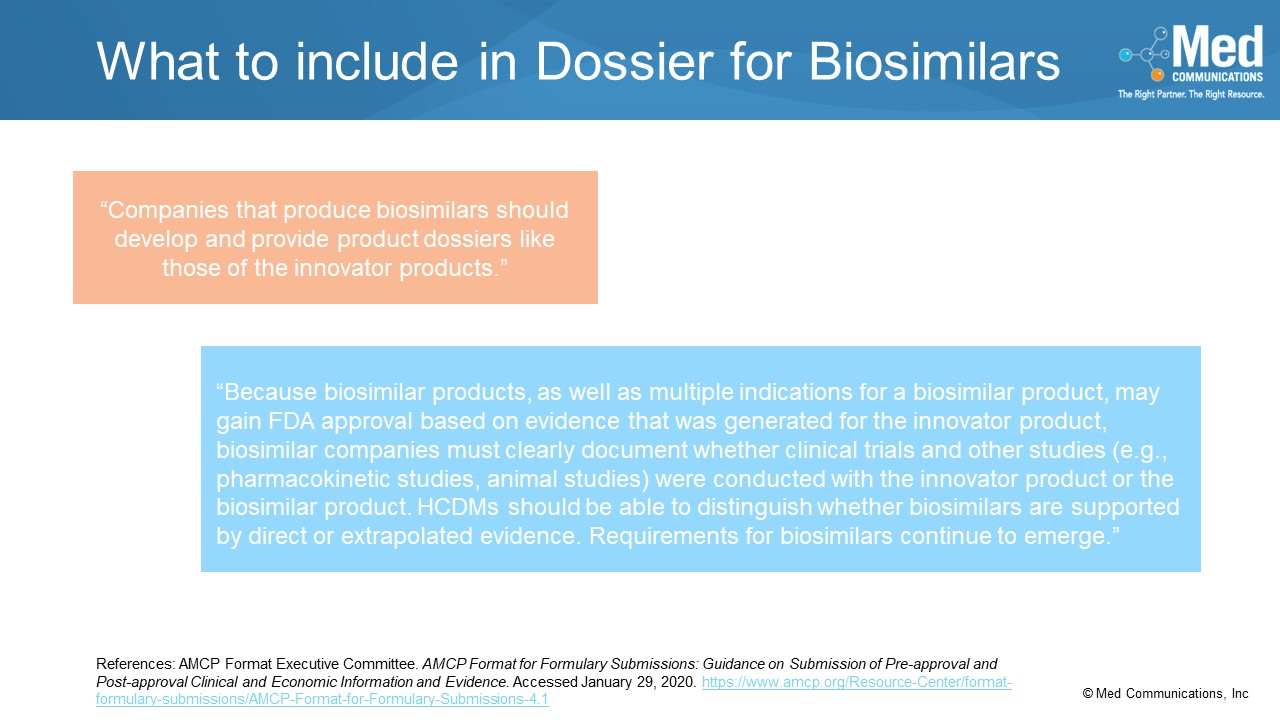
In the Special Content Considerations section of the AMCP Format for Formulary Dossiers, the fourth topic is Biosimilars. With the interest in biosimilars, health care decision makers need to know…









