Med Communications offers a boutique pharmacovigilance experience with global reach, combining personalized attention with international regulatory expertise. Seamless integration of pharmacovigilance and medical information improves workflow efficiency. Our approach combines innovation…

A pharmacovigilance (PV) system covering all PV activities is required of all marketing authorization holders. This system must include the activities of collection and processing of safety information, reporting to…

Every clinical trial requires safety monitoring and management, beginning early in the product development life cycle. The data obtained during safety monitoring help with product safety profile classification and also…

Pharmaceutical companies operate in a highly regulated environment, especially when it comes to global pharmacovigilance (PV) systems. Pharmacovigilance professionals play a crucial role in protecting patient safety while navigating complex…
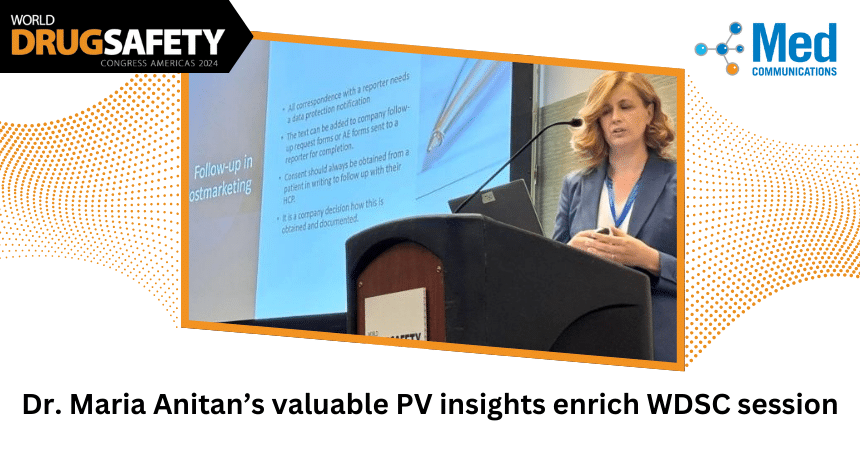
Important pharmacovigilance topics were discussed during the World Drug Safety Congress Americas in Boston last week, including valuable insights on data privacy shared by Med Communications’ Maria Anitan, MD. Some…

Med Communications has a remote-first, flexible business model that allows us to ensure business continuity for our clients regardless of what challenges may arise, including inclement weather and natural disasters….

Med Communications’ team of highly skilled pharmacovigilance (PV) professionals bring practical experience and diverse perspectives to the table, having worked with a variety of product portfolios including drugs, vaccine, cosmetics,…
Med Communications is a trusted global pharmacovigilance (PV) and drug safety partner offering full end-to-end PV services. We offer support in the following areas: Expert Pharmacovigilance Team Our team of expert…

The United States Food and Drug Administration (US FDA) is now accepting investigational new drug application (IND) individual case safety reports (ICSRs) submitted electronically via the Electronic Submission Gateway in ICH…

If you are attending the World Drug Safety Congress Americas in Boston October 18-19, be sure to check out the talk our global head of pharmacovigilance and drug safety, Dr….









