Tap into the vast experience of our pharmacovigilance professionals
Med Communications’ team of highly skilled pharmacovigilance (PV) professionals bring practical experience and diverse perspectives to the table, having worked with a variety of product portfolios including drugs, vaccine, cosmetics, medical devices, and combination products. Our PV specialists have medical, pharmaceutical, and other scientific backgrounds, and many held positions in sponsor organizations, MAH organizations, and PV regulatory authorities before joining Med Communications.
Our pharmacovigilance team features independent thinkers who help create tailored solutions for our clients and adapt to evolving needs of the industry. Backed by the latest applicable technology, our staff members provide flexible and compliant solutions for our clients, acting as true partners for your in-house teams.
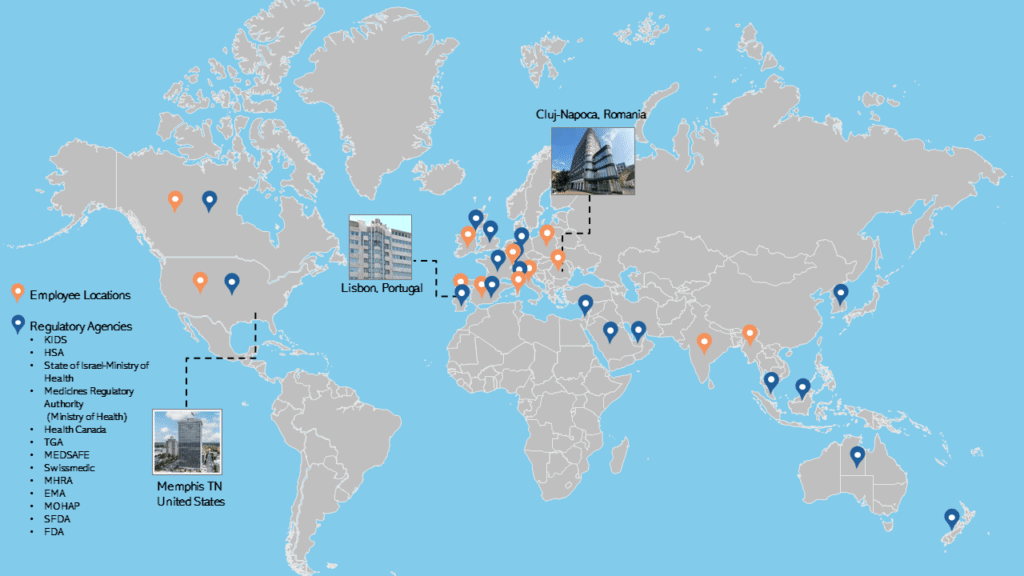
Med Communications works closely with clients to cover all aspects of the pharmacovigilance process, including individual case safety reports, periodic reports, signal detection activities, and reports to regulatory authorities. We have established pharmacovigilance systems in the US and many countries worldwide, including in the EMEA and APAC regions.
Contact us to discuss how we can meet your pharmacovigilance needs: 877-477-0977 or https://medcommunications.com/contact-us/.