
The 3-day Medical Affairs Professional Society (MAPS) Annual Meeting is March 8-11 in Miami Beach, FL, and Business Development Manager Stacy Witham will be attending. This meeting always has relevant…
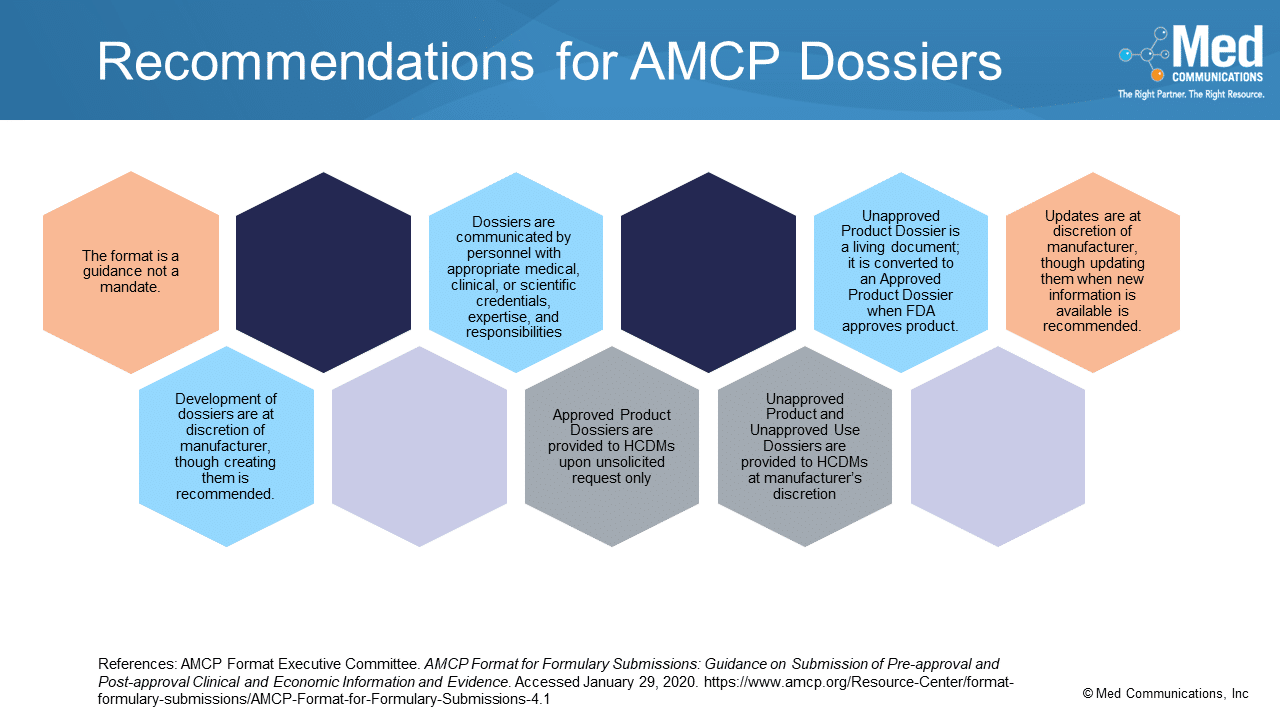
While many organizations follow the AMCP Format for Formulary Submissions, it is only a guidance, not a mandate, for how manufacturers and health care decision-makers can act in the process…

Rare Disease Day is tomorrow, February 29, 2020. According to rarediseaseday.org, over 300 million people are affected by rare diseases worldwide every day. Med Communications partners with many companies involved…

The two biggest differences between versions 4.0 and 4.1 of the dossier formats are the addition of the new types of dossiers (discussed in previous posts), and the importance placed…
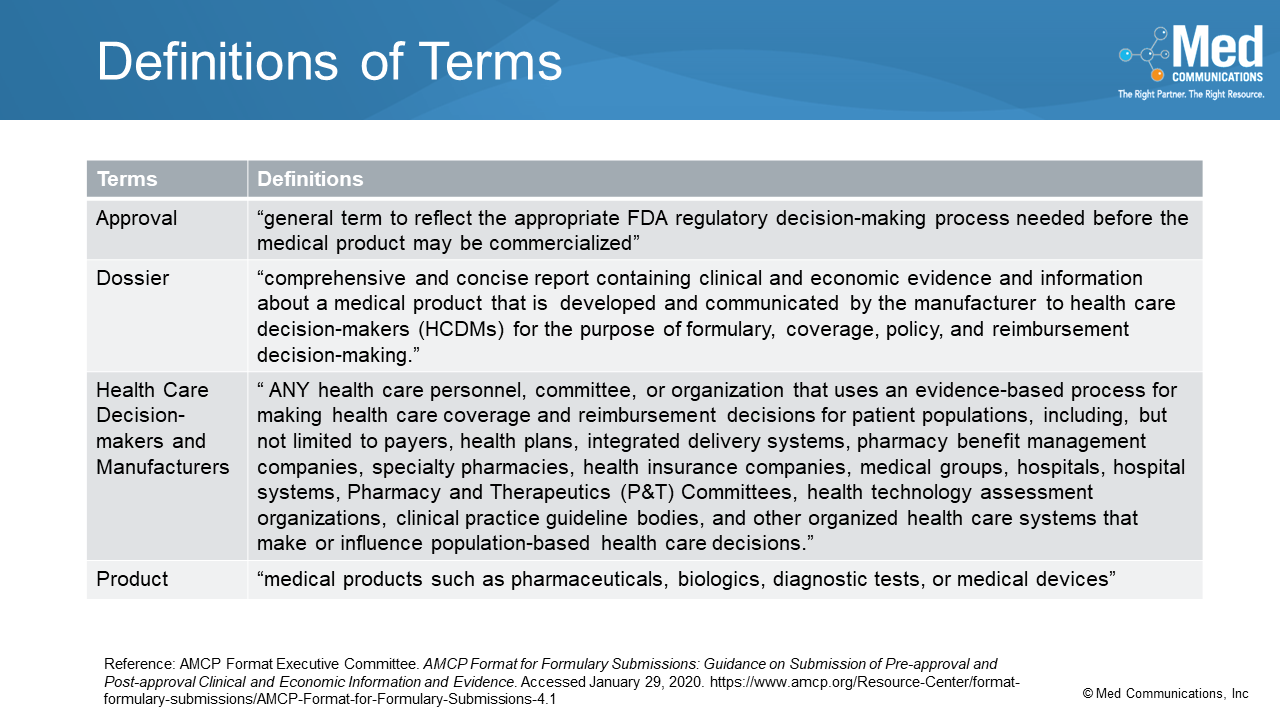
Some of the terms used in the AMCP format have been broadened with the new version. For example, “product” in past versions primarily meant pharmaceutical products, but that term has…
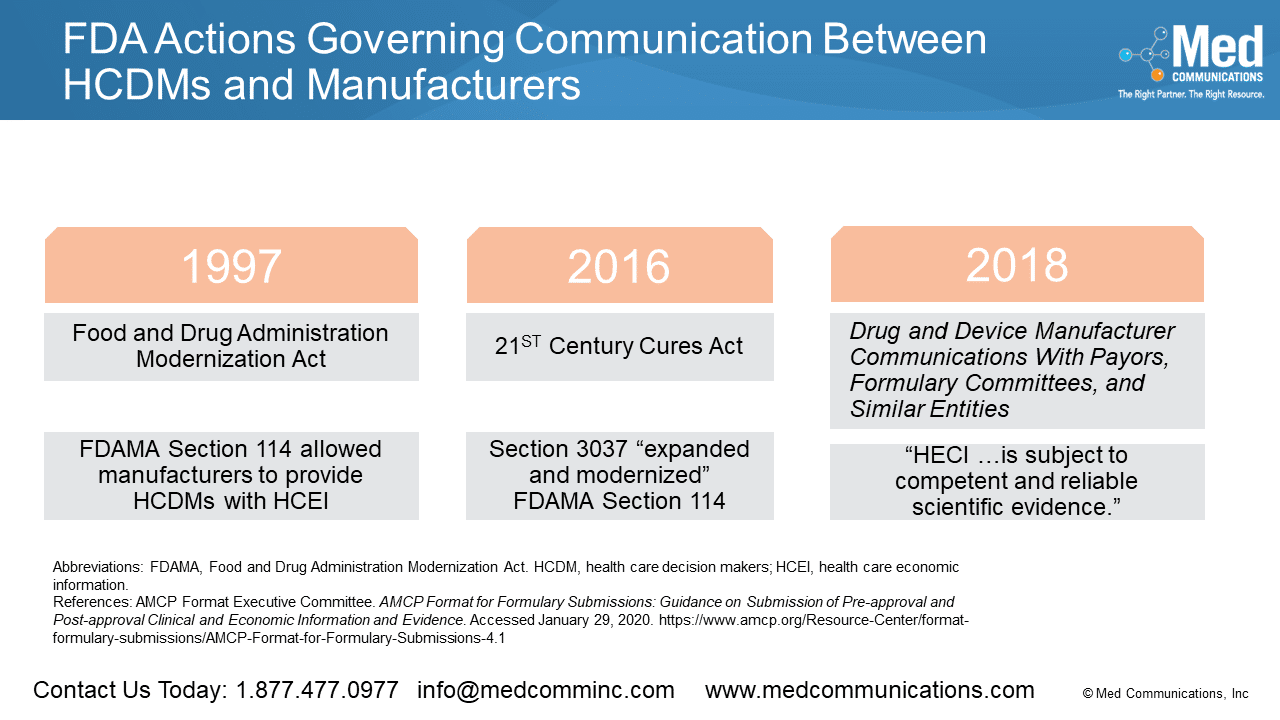
One driver for the new AMCP format was the FDA Final Guidance, Drug and Device Manufacturer Communications With Payors, Formulary Committees, and Similar Entities, which allowed for communication of information…

Maria Anitan, Med Communication’s Global Head of Pharmacovigilance and Safety, will be attending the 3-day Pharmacovigilance and Risk Management Strategies Conference, January 27-29 in Washington, DC. With topics such as…
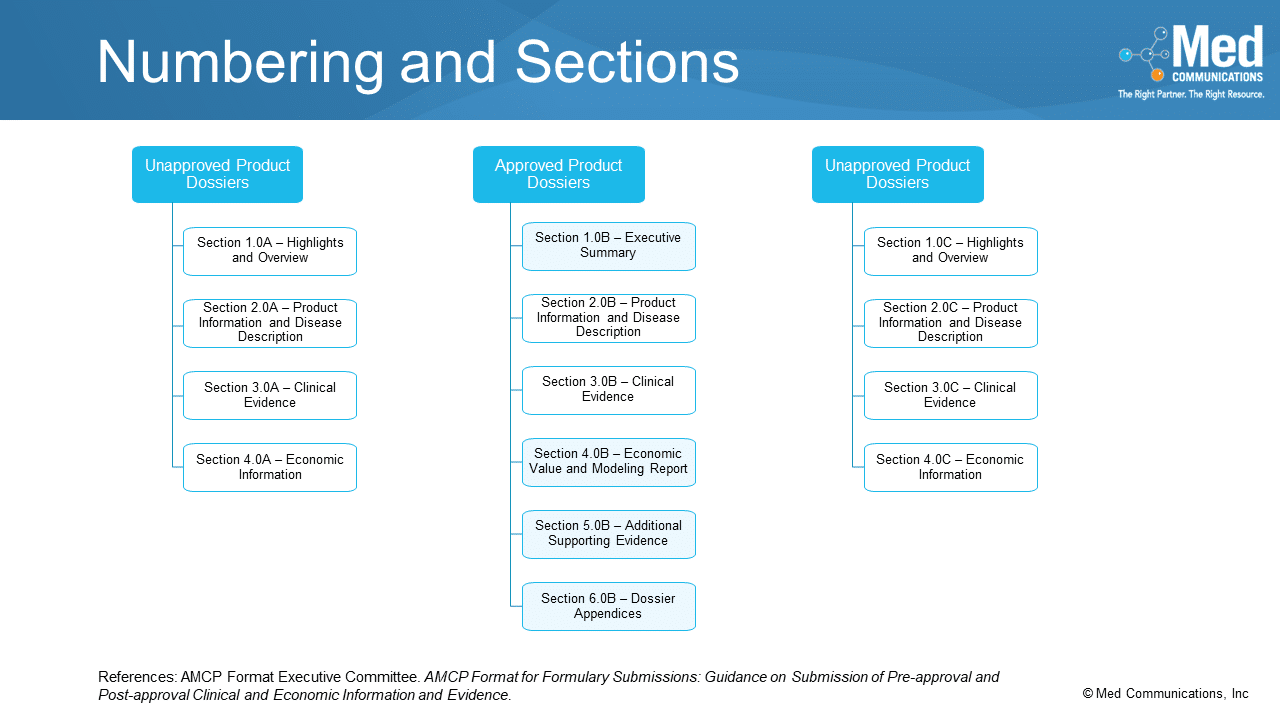
With the change to having three types of formulary dossiers in the new AMCP Format for Formulary Submissions, the numbering of the sections within each dossier changes to include an…
If you’re wondering how the new dossier format affects your company’s or your client’s formulary dossiers, you have a few options to get up to speed: ✓ Follow our page…

January 12 is National Pharmacist Day. Thank you to all of the pharmacists in our lives, including the many we work with at Med Communication, for the knowledge, skills, compassion,…









